#AskAChemist: What’s the deal with fabric dyes?

This week on Ask A Chemist…
@skepchicks @DrRubidium Why is wool dyed in acid, but cotton dyed in base? #AskAChemist
— Robert Coolman (@PrimeViridian) January 14, 2015
Short answer:

Longer answer:
Wool fibers are basically bundles of protein, specifically the protein alpha-keratin. All proteins are polymers of amino acids, have the general structure shown below.

Protein fibers like wool respond really well to so-called “acid dyes”. The dyes aren’t acids, but the acid dye’s salt version. Let’s take a look at a real example of an acid dye, beautiful acid red 73!
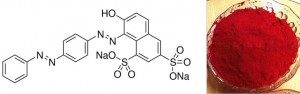
See where the sodiums (Na) are? When there are hydrogens in those positions, acid red 73 is an acid. With the sodiums there, it’s a salt. If we throw this salt in water, or a water-based solution, it’ll ionize to Na+ and dye-. The dye- is good, because we can make wool’s proteins have lots of positives spots for lots of dye- ions to stick to. See those ~NH~ groups in protein’s ? They can be protonated by an acid to become ~+NH2~. We can put wool in a water-based acid bath, add our dye salt, and let opposites attract*. Besides + and – hanging out, other intermolecular interactions keep the dye on the wool. Voilà! Chemistry! Dyed wool.

What about cotton? Like wool fibers, cotton fibers are also polymers. Cotton’s monomers aren’t amino acids, but glucose. Cotton is a polysaccharide, specifically, cellulose (below).

See all those ~OH~ groups of cellulose? They can be deprotonated by a base to leave ~O-~. These negative sites are attractive to dye+, which we can get by dissolving basic dyes (actually salts) in water or water-based solutions. Gosh, this sounds familiar… yep, it’s like the wool process, but opposite charges. With cotton, the fiber is made negative with base and attracts dye+. Thing is, using basic dyes with cotton requires the use of a mordant – another chemical that helps keep on the dye on the cotton fiber. Without a mordant, basic dyes don’t stay put like we’d want and can come out in the wash. Fear not, a whole host of cellulose specific dyes that stay put and don’t require mordants have been designed by crafty chemists.

_______Additional References______
Aspland, J. R. (1993) The Application of Ionic Dyes to Ionic Fibers: Nylon, Silk and Wool and Their Sorption of Anions. Text. Chem. Color., 25, retrieved from http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.405.4&rep=rep1&type=pdf
Aspland, J. R. (1997) Textile Dyeing and Coloration. Research Triangle Park: American Association of Textile Chemists and Colorists
Leon, N.H. (1972) Structural aspects of keratin fibres. J. Soc. Cosmet. Chem., 23, retrieved from http://journal.scconline.org/pdf/cc1972/cc023n07/p00427-p00445.pdf
____________________________
Featured image was made by the author using PowerPoint
Prince gif from rampages
General protein structure from UCLA’s Illustrated Glossary of Organic Chemistry
Acid red 73 structure from Sigma-Aldrich and dye powder image from Nuoou Chem
Carton gif from thechive
Cellulose structure from ngr.utk.edu
you’re welcome gif from tumblr