2D Molecules that Form Our 3D World
Disciples of organic chemistry know that aromatic compounds compose one of the most important classes of molecules. Very specific rules dictate the classification of these molecules, but one of the simple rules is that they are planar. By tacking on chemical functional groups to these 2D compounds some of the most well known 3D chemistry is formed. Keep reading below the fold for a good mix of organic chemistry with some fascinating applications that exist in your life everyday.
Also, a few months ago, I did an organic chemistry challenge. It was well received and here is another go around for you fellow O-Chem nerds, so keep reading until the end for those who want to play along. The answers will be posted next week.
Aromaticity is a well researched topics. Disclaimer: all of these rules can be broken, but generally aren’t. Some organic chemists have made careers by breaking the rules, so maybe a ‘Bad Boys of Organic Chemistry’ piece can follow if there is interest. For now, let’s stick to the basics.
1. These molecules are planar. This rule is simple enough.
2. Atoms that contribute to the aromaticity form a ring or rings.
3. These rings alternate single and double bonds.
4. There must be an even number of electrons that contribute to the aromaticity, but it cannot be a multiple of 4. Another way to determine this is that every double bond in the structure counts as 2 electrons.
Let’s do a super simple example that runs through our check list…
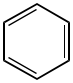
Here is benzene.
1. This molecule is planar. Molecules that break planarity are drawn with triangles and dashed wedges.
2. Benzene is a ring.
3. This ring alternates single and double bonds.
4. There are three double bonds, hence 6 electrons contributing to aromaticity. This is not a multiple of four.
Everything looks good according to the check list! Keep this in mind later. I will give you some structures and you can determine whether or not they are aromatic.
Alright, all of that is great. The next question is why are aromatic compounds important? The simple answer is they are the building blocks for some of life’s most prevalent compounds.
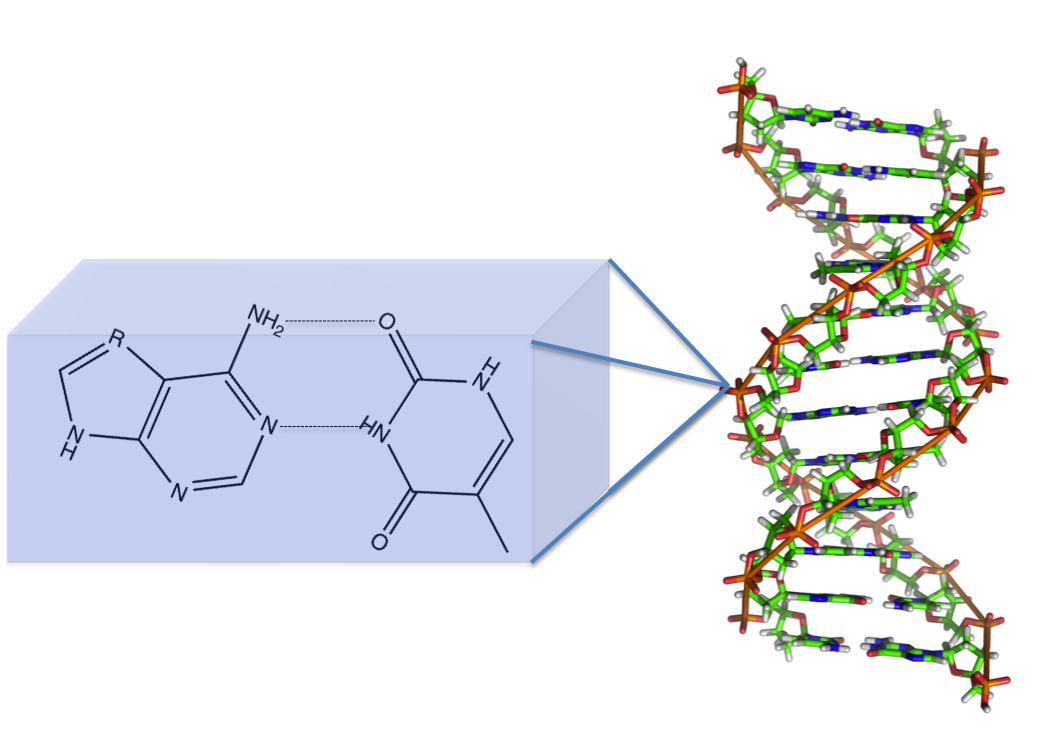
DNA anyone? The DNA base pairs: guanine, cytosine, adenine, and thymine all have aromatic elements. These aromatic groups are made unique with the addition of functional groups in specific arrangements generally including oxygen and nitrogen. However, it is the aromaticity of the DNA bases contribute to the overall stability of the double helix structure.
There are several intermolecular interactions contributing to the formation of this helix. The one you are most likely familiar with is hydrogen bonding between the base pairs (seen in the featured image). However, the one that is integral to this shape is intermolecular pi-pi bonding. Electrons are located in orbitals. For simple organic molecules, these orbitals are usually the sigma and pi orbitals. When a double bond occurs, these electrons are going into a pi orbital. The pi electron density can form favorable interactions with each other.
Compare it to building an Oreo tower, I know that I have built at least one of these (or many). In this hunger inducing metaphor, the cookie is the base pair and the cream is the electron cloud. When building a tower you can just take the oreos and stack them on each other, you need the cream to make it stick. DNA is the same way, you need the electron cloud to assist in stabilizing the system. Make sense?
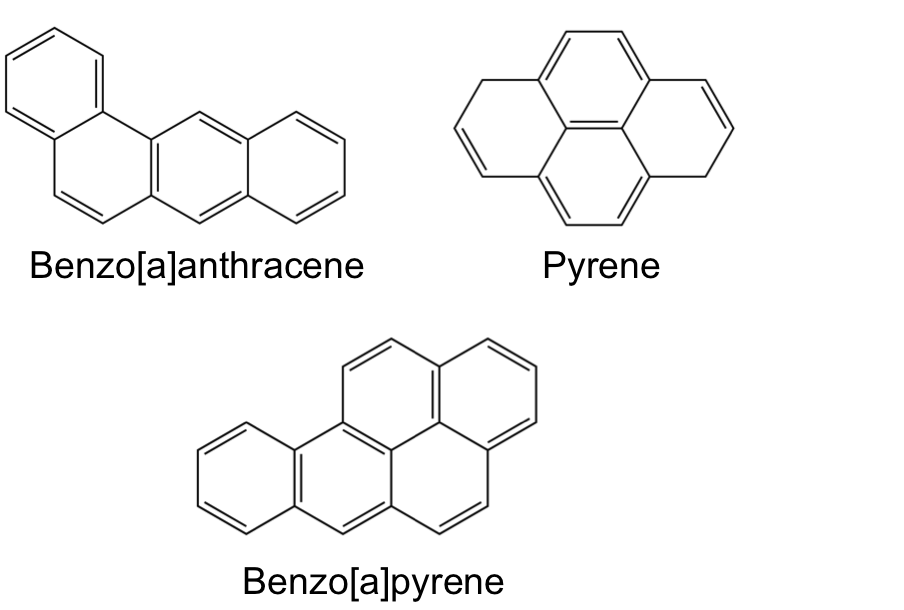
Now to give a completely different example of 2D molecules. There are a group of molecules named polycyclic aromatic hydrocarbons, PAHs for short. Just like DNA you encounter these everyday. These are products of oil, coal, smoke, etc. They are integral to combustion mechanisms. Also, the next time you enjoy your delicious meal off the grill, they are probably in that as well. Also, you can find them in space including the interstellar medium and comets. You get the idea, they are EVERYWHERE. Here are a few so you can visualize some examples.
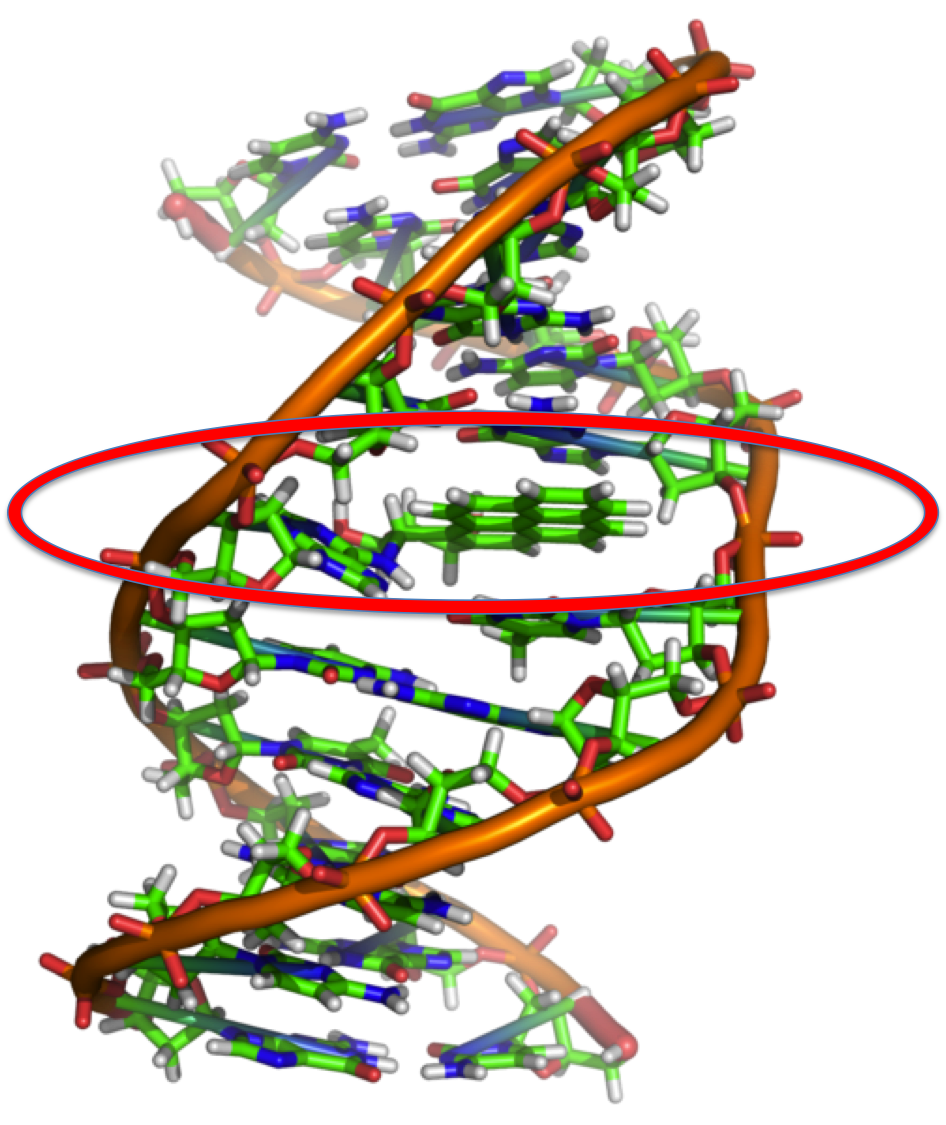
Just to further illustrate how 2D molecules can be mashed together. Benzo[a]pyrene is commonly intercalated into DNA. [Aside: This interesting problem is something that I have actually published so it is a bit of a pet application of mine.] This process is known to inhibit normal, everyday DNA processes and can ultimately lead to cancer. Benzo[a]pyrene can be ingested from cigarette smoke and easily slide into DNA due to structural similarity as a result of aromaticity. I circled the disrupted part of DNA in the figure below.
To end this aromaticity lesson — As promised there is going to be a simple three question challenge. It is simple, determine whether these compounds are aromatic or not? I will post the answers next week so leave them in the comments.
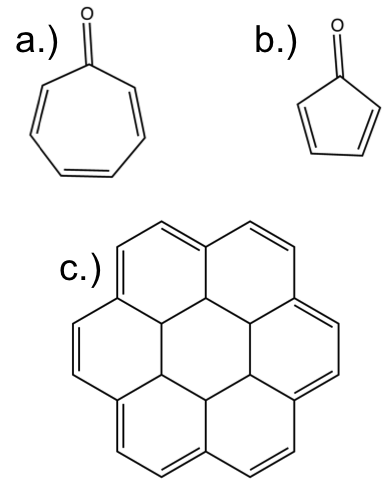







For “C” were you meaning to show [18]annulene, or are you being tricky? I’m not going to post answers since I probably have an “unfair” advantage so to speak, but “C” is interesting.
It is a distinct possibility that I am trying to be tricky.
Haha. Well, today while I was procrastinating setting up a DIBAL reduction, this made me chuckle a little bit. I was just a little surprised you’d be that tricky. I was fooled by something similar during my first organic graduate level course. :)
That was awesome– very interesting. I love articles like this by people who actually know what they’re talking about and aren’t just some armchair scientist.
I’m not entirely clear on whether rule 3 means that the alternation of single and double bonds must continue around the entire ring perimeter. The interpretation of this rule affects my answer on item a. Here I assume that the toggling must apply to the entire perimeter.
Answers:
a) Not aromatic, because bond alternation fails. (For a septagon, there is no possible solution that would fit that property due to the odd number of sides.)
b) Not aromatic. Bond alternation is incomplete, and having exactly two double bonds fails the “multiple of 4” rule.
c) Aromatic. It appears to pass all rules. Whatever molecule this is, it is extremely regular. Reminds me of honeycomb.
There is a hint that I didn’t offer to make it more challenging is: chemical structures can be drawn in more than one resonance form ( a different arrangement of electrons).
Nice article. It was a nice way to look at DNA
a) Aromatic
b) Not Aromatic
c) Aromatic
Oo, oo! Another quiz! Good one!
I say not, not, and aromatic, in that order.
a) aromatic
b) tricky – I’m gonna say no, but I have a sneaky suspicion I might be wrong here (I have a feeling I was tripped up by this one a few years ago in my advanced O-chem course, but I can’t remember how)
c) Also tricky, but no.
Answers?