The Adventures of Wintergreen Bottoms

 Naked and Famous Denim’s new sratch-n-sniff jeans smell minty fresh, stay clean thanks to Teflon®, and could make designer Brandon Svarc a mint. But… there is no mint in these bottoms. These dungarees owe their scent to wintergreen.
Naked and Famous Denim’s new sratch-n-sniff jeans smell minty fresh, stay clean thanks to Teflon®, and could make designer Brandon Svarc a mint. But… there is no mint in these bottoms. These dungarees owe their scent to wintergreen.
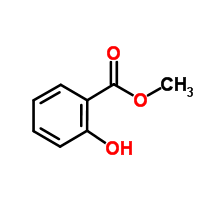
Though often grouped with the plants peppermint (Mentha piperita) and spearmint (Mentha spicata), wintergreen (Gaultheria procumbens) is not a member of the Methna crew (genus). Wintergreen may not be a mint, but the chemical responsible for its scent and flavor – methyl salicylate – is often described as “minty”. Oil of wintergreen, easily distilled from plant leaves, is 96-99% methyl salicylate and very pungent.
Oily jeans not being what Svarc was after, he used microcapsules to lock away the scent until scatched. Microcapsules, often easily broken polymer shells, are all the rage in cosmetics and offer a way to time-release all sorts of ingredients. These jeans’ microcapsules likely contain lab-made methyl salicylate in a formulation suitable for the design, manufacturing process, desired scent level, advertised scent life (10 washes), etc.
While we once used oil of wintergreen to scent and flavor, much of wintergreen is now actually methyl salicylate whipped-up in a lab. Good thing too! We use a LOT of methyl salicylate. We love our wintergreen mouthwash, toothpaste, gum, breathmints, shampoo, candy, medicinal creams/lotions/ointments (CLOs), etc. In fact, medicinal CLOs is often where wintergreen meets a mint. Or rather, where methyl salicylate meets menthol, the chemical in peppermint oil chiefly responsible for its smell, taste, and feel.
Feel? Oh yes! Peppermint’s menthol is a cooling chemical that when applied to our skin triggers a “oh, that’s cold!” response. Wintergreen’s methyl salicylate, on the other hand, is noted for it’s warming (“burning”) action. Put menthol together with methyl salicylate and you’ve got Icy Hot.
Methyl salicylate is warming… does this mean Svarc has made hot pants?! Probably not. The jeans’ microcapsules are nearly on the outside surface, so they can be scratched for sniffing. Plus, the amount of methyl salicylate in the microcapsules is likely enough to smell, but not enough to feel.
These pants smell fresh and look clean, but won’t literally make the wearer hot. That’s next year’s scarf-n-sniff-n-sensation pants!
_____________
Featured image is from HelloGiggles
OutKast ‘So Fresh, So Clean’ gif from The Smoking Section
Methyl salicylate image from Chemspider





I am mid semester in organic chem II, so alas I find this very entertaining. :) On a sidebar, I once had toothpaste explode in my pocket, there was definitely a burning sensation that ensued mid morning that I will never forget and still laugh about. :)
Personally, I really don’t like the taste. Finding non-mint-flavoured toothpaste is really difficult.
This has to be a joke right? It has to be, how could anyone think that their pants should smell like something you associate with throwing up (Pepto Bismol) or having bad breath (breath mints).
Also, do they not realize that a certain percentage of their customers likely go commando. While they claim you won’t be able to feel it, I smell a lawsuit (see what I did there?) when a hot, humid day starts the old giblets to cookin’. And I don’t think wintergreen would be an appropriate condiment for that Hot Pocket.