2D Molecules in our 3D World — Answers!

I sincerely apologize for delaying the answers to the quiz proposed in the ‘2D Molecules that Form Our 3D World‘ post. (I have been scrambling preparing for chemistry conference season and helping design sloth t-shirts for Women Thinking, inc.) If you still haven’t read the post and taken a stab at the questions, then check it out before reading below the fold. Congratulations to commenter notidealist for being the first and only commenter to correctly answer the quiz.
The answers to the post are:
a.) aromatic
b.) anti-aromatic
c.) aromatic
The resonance structures make each one of these a bit clearer that it is aromatic or anti-aromatic. Try ‘pushing’ the electrons to get a clearer view.
On the road this weekend, but let me know if there are any lingering questions in the comments. 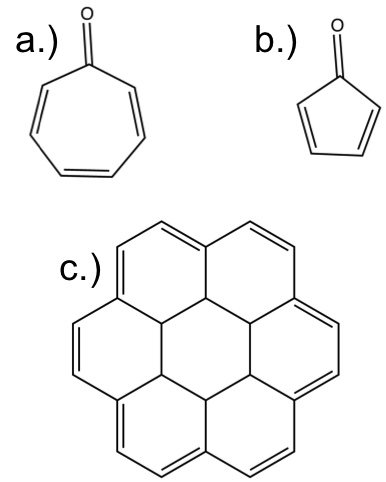





Doh!
I really need a hint regarding that first one – does keto-enol tautomerism play a part there?
Yes, that is exactly what happens.
OK, tropylium cation, I see! Sweet!
Also thujaplicin, Formosan cedar oil, and colchicine – important natural products.
Only a chemist could fully understand – I think it’s called a geekgasm.
I mean, I hope that comment wasn’t too “off”, I’m trying to say that those 7 membered ring structures are inherently exquisitely beautiful.
The aromaticity makes them more so somehow, with all the implications in terms of acidity, substitution, metal binding, diazotisation (I love diazotisation), ra ra.
I had forgotten all this and I want to thank you for reminding and inspiring me.
I’m going to object to “C” as being “not-necessarily aromatic”. I believe there is a paper that Carey and Sundburg’s part A cites that includes the aromatic version of this compound, but it definitely specifies the orientation of the hydrogens in the center. However, the compound also looses its planar configuration if the hydrogens in the center are not in this configuration, with the most drastic that I could model being with only 1 hydrogen different from the other 5. It almost bends into a taco shape with that configuration.
It may be that when not otherwise noted the assumed isomer is that of the aromatic one, or it might even be that aromaticity is so favored here that there is some kind of sigmatropic-like rearrangement that happens if it ever forms in a non-aromatic arrangement, but I just figured there was the possibility that without the stereochemistry being defined in the middle, there was the possibility of the problem being ambiguous.
*I’m going to object to “C” as being “aromatic” and say that it’s “not necessarily aromatic”. (to clarify since I didn’t write that very clearly)
Thanks for the helpful information.