Diels-Alder Puzzle Answers

About a week ago, I proposed two organic chemistry puzzles in my post Dear Diels & Alder. As promised, here are the answers to the puzzle. We did have one brilliant reader, Jack99, that answered the puzzle correctly (although it seemed that several other commenters knew the answer as well).
The puzzle questions are in the link above if you want to take a look before reading the answers.
Puzzle 1 Answer:
In this puzzle, I gave you the full reaction and asked you to fill in the arrows. The diene is colored red in the reactant and product. The dienophile is colored dark blue in the reactant and product. The two green arrows are pointed to nothing because they are both forming a new bond that are represented by black in the product. The light blue arrow is forming the double bond in the product.
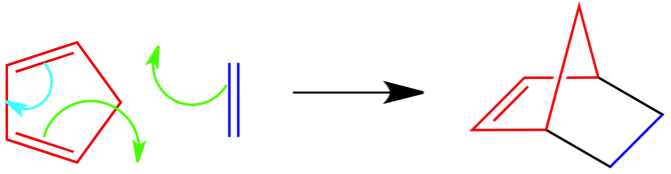
Puzzle 2 Answer:
This puzzle was more difficult than the first, but similar. This time you had to guess the product AND place the arrows in the correct place. Similar to the above answer, red is the conjugated diene and dark blue is the dienophile. The green arrows are forming the new bonds and are shown in black in the product. Again, the light blue arrow forms the double bond.
Did all of you playing at home guess the answers correctly!? Hopefully, these 3D puzzles were fun. I was excited to see so much organic chemistry enthusiasm from the commenters. Let me know if there are still any questions about the puzzle answers. Again, congratulations to Jack99 for guessing the answers correctly!

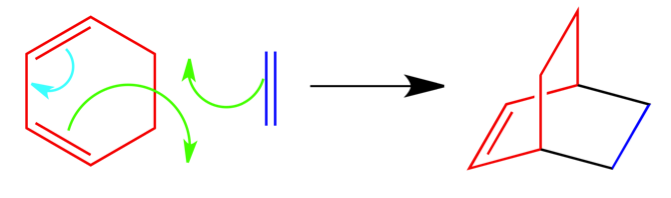




I really enjoyed these puzzles. The most advanced chemistry I ever took was the college course required of all engineers at my school. Getting the chance to try my hand at some simple organic chemistry was fun.
I would love to see more posts like these, perhaps from other scientific disciplines, as well.
Yay, I did it! Personally, my favorite organic reaction is the electrophilic aromatic substitution.
Aw, shucks, Jacqueline, Thanks, but it’s you who are brilliant!
Hope I didn’t spoil it too much by giving the game away – I read Amy’s post “please post the answers” as being from you and got carried away with enthusiasm.
Please do this sort of thing again.
Aww gee, thanks, Jack.
I will see what I can do about putting together some other chemistry puzzles (maybe an electrophilic aromatic substitution next time). Then I will stretch my brain to put together something in another scientific discipline as well. Glad so many people had fun with it!
Yes! Please more of this! Really appreciate it and hooray for Jacqueline!
Here is Diels-Adler in brick via Jennifer Ouellette: http://cen.acs.org/articles/90/i2/Diels-Alder-Brick-Countering-Veggie.html
This was highly enjoyable! A funny and a little ironic fact is that in organic chemistry (carbon-based chemistry) the carbon-carbon bonds are among the most difficult to produce. Diels-Alder is, as we have seen, one way to achieve them but it is limiting in what you can do. The Grignard reaction is another for which Grignard received the 1912 Nobel Prize in Chemistry. 2010 Nobel Prize in Chemistry was given “for palladium-catalyzed cross couplings in organic synthesis”. This reaction is a milestone in organic chemistry and a lot of compounds would not be possible to produce without it. So it seems if you want to win a Nobel Prize a good bet is to find a new carbon-carbon bond producing reaction and of course Diels and Alder got a Nobel Prize in Chemistry in 1950 for their reaction.
Yay for Chemistry! And Diers Alder is also my favorite organic reaction. Then again, I was lucky enough to have one of the best teachers ever in all my organic chemistry classes (which were many). One of the most fun projects I did with him was catalizing Diers-Aldes type reactions in high pressure-low temp, which was basically putting the reactants in a flexible tube inside a sealed compartment filled with water and then frozen at -20ºC. It was the first time I heard of reaction volume as an important aspect of chemistry, usually you just throw everything in solution and let it be…